Statistical Analysis Plan (SAP)
HYDRATION TO OPTIMIZE METABOLISM
Principal Investigator
Olle Melander, MD, PhD
Professor of Internal Medicine
Lund University and Skåne University Hospital
Malmö, Sweden
Protocol identification number
H2O Metab Proposal (dated 2017-12-15)
ClinicalTrials.gov identifier
2016894
Author
Tommy Schyman, BSc
Statistician
Clinical Studies Sweden – Forum South
Skåne University Hospital
Lund, Sweden
Version
Draft 0.3

SIGNATURE PAGE
Principal Investigator
__________________________
Olle Melander (2017-12-15)
Author
___________________________
Tommy Schyman (2017-12-15)
Abbreviations
ACTH
Adrenocorticotrophic hormone
CI
Confidence Interval
DBP
Diastolic Blood Pressure
eGFR
Estimated glomerular filtration rate
HbA1c
Glycated hemoglobin
HDL
High Density Lipoprotein
IQR
Interquartile Range
ITT
Intention-to-Treat
LDL
Low Density Lipoprotein
OGTT
Oral glucose tolerance test
OR
Odds Ratio
PP
Per Protocol
RCT
Randomized Clinical Trial
SAP
Statistical Analysis Plan
SBP
Systolic Blood Pressure
SD
Standard Deviation
VP
Vasopressin
Table of contents
1. Introduction ................................................................................................................................. 5
2. Study design ................................................................................................................................ 5
2.1 Sample size calculation ........................................................................................................ 6
3. Aims and objectives ..................................................................................................................... 7
4. Outcomes .................................................................................................................................... 7
4.1 Primary outcome ................................................................................................................. 7
4.2 Secondary outcomes ........................................................................................................... 7
4.3 Safety outcomes .................................................................................................................. 7
5. Populations and subgroups to be analysed ................................................................................ 8
5.1 Populations .......................................................................................................................... 8
5.2 Subgroups ............................................................................................................................ 8
6. Analyses ....................................................................................................................................... 8
6.1 Primary outcome ................................................................................................................. 8
6.2 Secondary outcomes ........................................................................................................... 8
7. Missing data ................................................................................................................................ 9
1. Introduction
The aim of this project is to test in a single-centre randomized clinical trial (RCT), if water
supplementation in subjects with high plasma levels of vasopressin (VP) (measured by a stable VP
marker of its precursor hormone called “copeptin”) can reduce fasting levels of glucose (primary
outcome measure), risk of new-onset diabetes and other cardiometabolic risk factors (secondary
outcome measures).
This statistical analysis plan (SAP) will give more detailed descriptions of the endpoints in the study
and the corresponding analyses.
2. Study design
Study subjects will be recruited from 4 ongoing population studies in the Scania region encompassing
altogether approximately 20 000 individuals within the current age span. Copeptin will be measured in
-80 degree frozen plasma samples from these 4 studies. Individuals having a copeptin concentration of
>6.1 pmol/L (women) or >10.7 pmol/L (men) will be invited to participate in the screening and inclusion
process of this study. If fewer than expected will be recruited from these 4 studies, employees of the
City of Malmö and Skåne University Hospital who are 20-75 years will be invited to undergo a fasting
plasma determination of copeptin. The same cut-off values will be used for invitation to the study. If
more study subjects are needed, a third source of recruitment will be advertisements in local press.
The study is a parallel-group RCT with two arms during 12 months. Subjects will be randomized to the
water-intervention (1.5 L total in three (3) 0.5 L increments daily on the top of habitual intake) and
control group (1:1). The randomization will be stratified by gender to pursue equal distribution of
intervention and control group for both male and female subjects. Both groups will receive general life
style advice (general oral and written advice on diet and physical activity). Smart bottles, which are
volume sensitive and can be linked to an Android or iPhone application for individual monitoring
purposes will be provided to subjects in the active treatment arm.
Clinic visits are performed at 8 time points: visit 1, visit 2, baseline, 3 weeks and 3, 6, 9 and 12 months
at which cardiometabolic risk factors and hydration parameters are measured. The study design is
visualised in Figure 1 below.
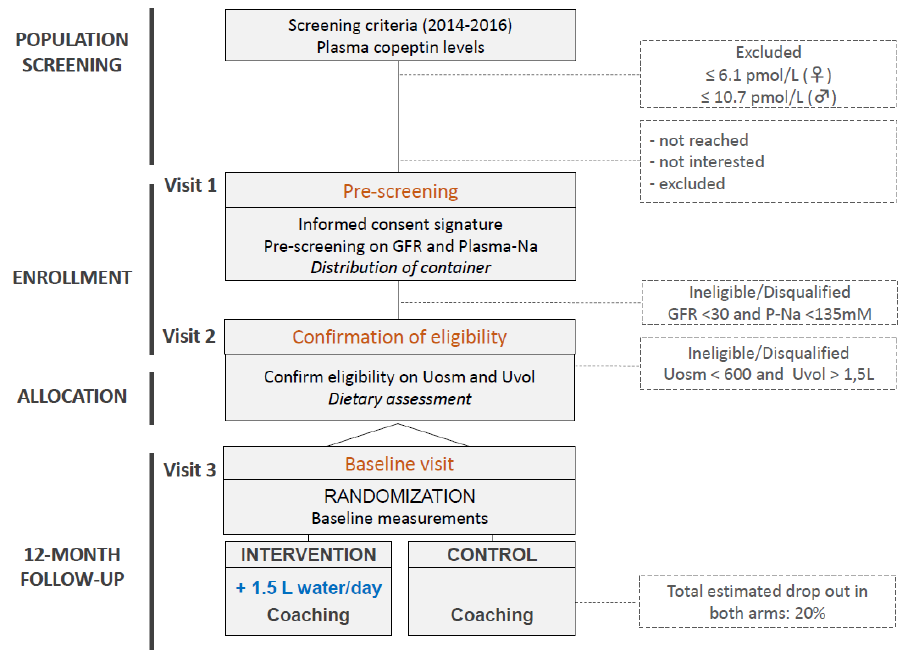
Figure 1 Flowchart of screening and inclusion process
2.1 Sample size calculation
The primary outcome measure for the power calculation is the difference between active and control
treatment in the change of fasting plasma glucose between baseline and 12 months. We use prior effect
estimates from the largest RCT for diabetes prevention study in Europe, i.e. the Finnish Diabetes
Prevention Study (FDPS) (New Engl J Med 2001;344:1343-50), which compared individual life style
counselling (active treatment) with general oral and written life-style advice (control treatment) in
relation to risk of new onset type 2 diabetes and change of plasma glucose concentration and found a
58% decreased relative risk of diabetes. After 12 months, the fasting glucose in the active treatment
group was reduced by 4±12 mg/dL vs a 1±12 mg/dL increase in the control group with the mean
difference of 5 mg/dL of the 12-month change of fasting glucose being highly significant (P<0.001).
To obtain sufficient statistical power to detect a clinically significant effect size, we base our power
calculation on an effect of water vs control that is at least 50% of what is considered an epoch changing
effect of life style, i.e. the difference observed in the FDPS, while assuming the standard deviation for
the change (s = 0.67 mmol/L) as observed in FDPS. In order to be able to detect ≥50% of that effect (a
difference of 2.5 mg/dL = 0.14 mmol/L between treatments in 12-month change) we need 319 subjects
in both the active and control treatment groups at a power of 80% and a 2-tailed significance level of
<0.05. Experiences from the WIT (see above) indicate 8-10% lost to follow-up during 12 months. As
our study subjects are “healthy subjects at risk” rather than patients, we anticipate a higher drop-out rate
(up to 20%). Based on the power calculation (n=319+319) we will enrol 400 individuals in each
treatment group (n=400 in active and n=400 in control arm), i.e. a total number of 800 individuals, to
have a final sample size robust to lower compliance than anticipated.
3. Aims and objectives
To study if water supplementation in subjects with high plasma levels of VP (measured by a stable VP
marker of its precursor hormone called copeptin) can reduce fasting levels of glucose, risk of new-onset
diabetes and other cardiometabolic risk factors.
4. Outcomes
This section will present the outcomes investigated to answer the study aims and objectives. The
analyses are described in section 6 Analyses.
4.1 Primary outcome
Fasting plasma glucose. It will be measured at baseline, 6 months and 12 months.
4.2 Secondary outcomes
Diabetes incidence
New-onset diabetes is defined as 2 consecutive fasting plasma glucose values ≥7.0 mmol/L or 2-hour
post glucose challenge value of ≥11.0 mmol/L at an oral glucose tolerance test (OGTT). In addition,
new onset diabetes is considered present if diagnosed by a physician outside of the current study, as
assessed in the questionnaire (answering yes on having had a physician diagnosis of diabetes or having
been prescribed medication for diabetes).
Cardiometabolic risk factors
2-hour glucose during OGTT
HbA1c
Waist circumference
Body mass index
Systolic and diastolic blood pressure
Serum triglycerides
HDL- and LDL-cholesterol
Apo-B
Apo-A1
Cortisol
Adrenocorticotrophic hormone (ACTH)
Insulin (fasting and 2h post OGTT)
Glucagon (fasting and 2h post OGTT)
C-reactive protein
Estimated glomerular filtration rate (eGFR)
Creatinine clearance
All these risk factors will be measured at baseline, 6 months and 12 months.
Other blood laboratory parameters
Copeptin, Sodium, Potassium, Urea and Erytrocyte Volume Fraction (%).
Urine laboratory parameters
Volume, Osmolality, Creatinine, Sodium, Potassium, Urea, Albumin/creatinine ratio and Cortisol.
Surveys
Health-related quality of life, fluid/water and dietary intake (using Riksmaten 2010), stool form (Bristol
Stool Scale).
4.3 Safety outcomes
Adverse events
Adverse events are reported at each clinic visit.
Concomitant medications
Usage of medications during study period will be recorded.
5. Populations and subgroups to be analysed
5.1 Populations
Intention-to-treat (ITT)
All randomised study subjects. This will be seen as the primary population for the analysis.
Per Protocol (PP)
All randomised study subjects completing the whole study period (complete cases). For a specific
analysis, study subjects with missing data on any of the variables in the model will be excluded from
the analysis. Analyses of this population is seen as a sensitivity analysis to investigate whether
conclusions are sensitive to assumptions regarding the pattern of missing data.
5.2 Subgroups
Six subgroups will be analysed. All subgroups will be analysed using both ITT and PP populations.
High-high
All randomised study subjects having copeptin concentration above the previously specified cut-off
values, 6.1 pmol/L (women) or 10.7 pmol/L (men), at both population screening and baseline visit.
Top tertile
All randomised study subjects having copeption concentration in the top tertile (gender specific) at
baseline visit.
Diabetes mellitus
All randomised study subjects will be divided into two subgroups according to having diabetes
mellitus or not at baseline visit.
Gender
All randomised study subjects will be divided into two subgroups according to gender.
6. Analyses
All outcomes will be presented using descriptive statistics; normally distributed data by the mean and
standard deviation (SD) and skewed distributions by the median and interquartile range (IQR). Binary
and categorical variables will be presented using counts and percentages. SAS 9.4 will be used for all
statistical analysis.
The subsections below will describe analyses in addition to the descriptive statistics.
6.1 Primary outcome
The primary analysis will compare intervention groups (water supplementation vs control treatment) on
their mean change in fasting plasma glucose between baseline and 12 months using a linear mixed
model. Difference in fasting plasma glucose from baseline to time points where it is measured during
the study (6 and 12 months) will be the dependent variable. Study subjects will be considered as random
effects, treatment group and visit number as fixed effects. Baseline value of fasting plasma glucose will
be included as a covariate. The estimated difference in mean change from baseline to 12 months and the
corresponding 95 % confidence interval (CI) will be presented.
6.2 Secondary outcomes
Cardiometabolic risk factors will be analysed using the same method as for the primary outcome,
including usage of the baseline value for the actual factor as a covariate. Diabetes incidence will be
analysed using logistic regression, the odds ratio (OR) including 95 % CI will be presented.
In addition, correlation(s) between change in copeptin and change in cardiometabolic risk factors, other
blood laboratory parameters and urine laboratory parameters may be calculated.
7. Missing data
When analysing using the ITT population, model based multiple imputation (MI) will be used for both
primary and secondary outcomes. The number of imputations will be the largest value of:
10
the proportion of missing values for the actual variable * 100
200 burn in iterations will be used for all analyses. Trace plots and distribution plots will be created to
check the accuracy of the imputations.
